Abstract
BACKGROUND: Invasive fungal infections (IFIs) are a leading infectious cause of morbidity, mortality, and Health Care Resource Utilization (HCRU) in patients with hematological malignancies. Patients with at higher risk of acquiring IFIs are those with acute leukemia (AL), myelodysplastic syndrome (MDS) or undergoing hematopoietic stem cell transplant (HSCT). Needed to optimize antifungal use, Guidelines rarely include this group of patients, and more effort is needed to optimize the use of antifungal.
AIMS: The aim of this study was to demonstrate the potential benefits of the implementation of an antifungal stewardship (AFS) program in a Spanish Hematology-Oncology Department, in a tertiary care center. To test the long-lasting effects of a 2 years antifungal stewardship (AFS) pilot project, we analyzed and compared antifungal prescriptions regarding efficacy, IFI incidence, adaptation of prescriptions to current protocols, antifungal consumption and costs saving before and during the project
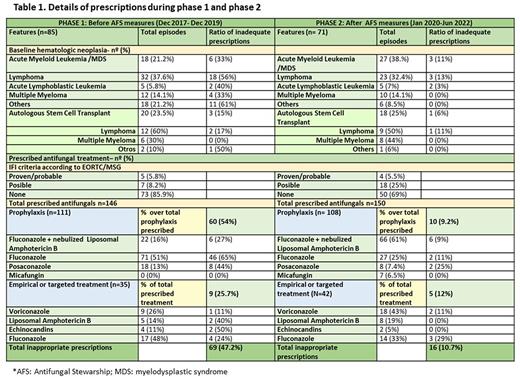
METHODS AND PATIENTS: This single-center comparative study included 156 inpatients ≥ 18 years, receiving systemic antifungals for prophylaxis or therapy of IFI for over four years: 85 in a phase 1 (December 2017- December 2019) and 71 in a phase 2 (January 2020-June 2022). In January 2020, we have designed a protocol for the use of antifungals in hospitalized patients based on the ECIL guidelines and according to local susceptibility patterns and, approved by the antimicrobial therapy team.
First line antifungal prophylaxis for high-risk patients on our unit is nebulized Liposomal Amphotericin B 20 mg 3/weekly combined with fluconazole 400 mg po/iv qd (salvage therapy: Posaconazole 300 mg tablet qd). Patients undergoing an autologous-HSCT and other medium risk patients receive fluconazole monotherapy 400 mg po/iv qd as primary prophylaxis during the neutropenic period. Front-line therapy for possible (empirical antifungal therapy) and proven/probable IFI (targeted therapy) is Voriconazole 2x6 mg/kg on day 1 then 2x4 mg/kg (salvage therapy: Liposomal Amphotericin B 3 mg/kg/day). A comprehensive mould-directed diagnostic approach was established combining microbiological work-up (cytology, culture & Galactomannan screening) and CT imaging.
From that date on, AFS included medical training (short sessions), a poster with main recommendations regarding antifungal treatment, and daily pharmaceutical counselling onwards.
RESULTS: In total, 156/1670 (9.4%) patients received antifungals. In phase 1, 182 antifungals were prescribed (76% for prophylaxis, 21.2% for empirical and 2.8% for targeted therapy) and 150 in phase 2 (72% for prophylaxis, 24.7% for empirical and 3.3% for targeted therapy). The number of prescriptions classified as inappropriate decreased from 47.2% to 10.7% during implementation of the AFS-period Antifungal prophylaxis main reasons for the inappropriate prescriptions were (phase 1/phase 2): lack of indication (44.5%/1.3%), wrong dose (4.4%/1.3%), and potential severe drug-drug interaction (12.1%/4%). Regarding antifungal therapy: drug choice (7%/0%), dosage (5%/1%) and potential severe drug-drug interactions with concomitant therapy (7%/2.7%) were the main reason for inadequate prescriptions (details are showed in table 1). Associated costs for antifungals accounted in phase 1 for 92.664€ (65.192€ for prophylaxis and 27.472€ for therapy) while in phase 2, from January 2020 to January 2022, were 58.274€ (33.883€ for prophylaxis and 24.391€ for therapy).
CONCLUSIONS: Our results demonstrate that the implementation of a regulated protocol would lead to a decrease of inappropriate prescriptions of antifungals with a drastic reduction of prescriptions associated to potential severe drug-drug interactions and medication errors or lack of indication. In addition, we could show economic savings, a total of 34.390€ in two years, without a significant increase in the diagnosis of IFI in critically ill patients (0.5% in phase 1 versus 6% in phase 2).
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal